There is a growing need for sustainable and effective energy storage options as the globe electrifies and implements renewable energy innovations more quickly. The anode and the cathode are two crucial parts that determine a battery’s characteristics and efficiency, and lithium-ion batteries are leading the way in this transformation.
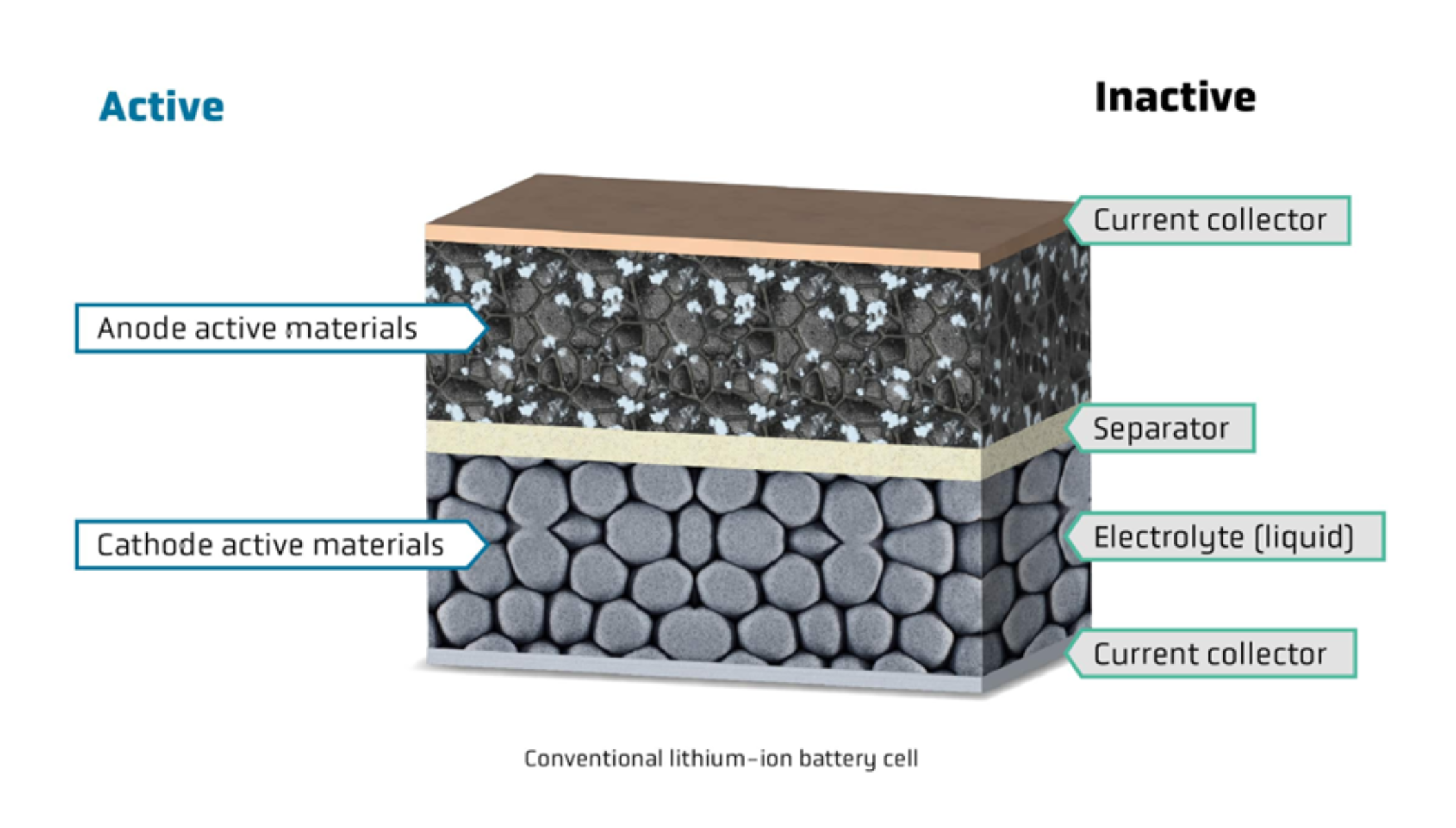
The chemicals that participate in the battery’s electrochemical processes are known as active materials. Throughout charging, the active component takes up ions, which it then eliminates during discharging. Energy may be stored and released in the battery because of to this ion exchange reaction method.
The battery’s efficiency, capacity, longevity, and performance all depend on its active components. The two electrodes that allow electric charge to flow in a battery or electrochemical device are called a cathode and an anode. Anodes are the negative electrodes where oxidation occurs, which means loss of electrons, while cathodes are the positive electrodes where reduction occurs, which means gain of electrons, exactly opposite from anodes. Metal oxides are commonly used as cathode compounds (CAM) throughout the charging cycle. Lithium cobalt oxide (LiCoO2), lithium manganese oxide (LiMn2O4), lithium iron phosphate (LiFePO4 or LFP), and lithium nickel manganese cobalt oxide (LiNiMnCoO2 or NMC) are the most often utilized cathode materials in lithium-ion batteries.
The energy density, affordability, and thermal properties i.e., thermal stability, of each of these materials differ. Electrons go from the cathode to the anode in a battery, saving energy that can be utilized to power gadgets in the future.
Conversely, anode active materials (AAM) are often composed of carbon-based materials such as silicon, graphite, or a mix of the two. Because of its solid arrangement, cheap price, and good electrical conductivity, graphite is the most often utilized anode material. Despite having a higher energy density, silicon anodes have problems with volume expansion as well as a lower cycle life.
Electrolyte is another important component that also contributes to the performance of the batteries. Solvent regimes that let salts separate into ions and allow for ionic conductivity inside batteries are referred to as electrolytes. These may consist of a variety of organic solvents, including ethers and carbonates, which are chosen for their electrochemical stability, viscosity, and dielectric constant in order to maximize productivity for uses such as Na-ion batteries.
https://www.purplan.com/en/glossary/term/active-materials
https://aquametals.com/recyclopedia/lithium-ion-anode-and-cathode-materials/
![]()